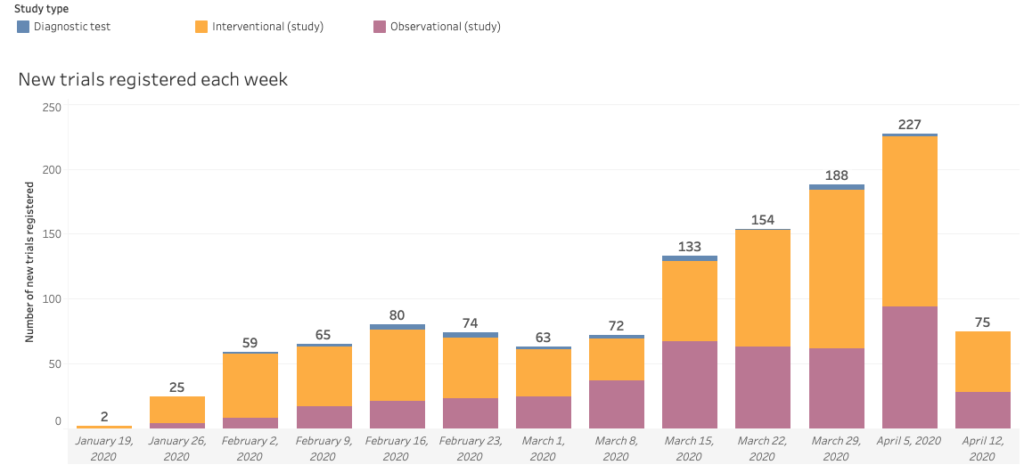
The number of clinical research trials for COVID-19 has rapidly grown from its initial detection in December of 2019 in Wuhan, China. Each and every day, scientist and pharmaceutical companies are working together to increase the number of clinical trials in order to find a vaccine sooner than later. If you are interested in participating in a trial, please fill out our survey here.
Potential New Treatment for COVID-19 Uncovered by BenevolentAI Enters Trials
BenevolentAI, a startup which has raised $292 million to apply AI to create drugs faster, today says it has uncovered an already approved drug as a potential treatment for COVID-19, after it applied its AI platform and team to the problem. The revelation, which has now appeared in peer-reviewed scientific journals and has entered clinical trials with a major pharmaceutical company, could offer a glimmer of hope to a world locked down by the pandemic.In February, BenevolentAI set up a specialist scientific team and launched an investigation using its drug discovery platform.
Baroness Joanna Shields, CEO of BenevolentAI, explained: “In response to the COVID-19 global health emergency, we turned our AI drug discovery and development platform toward understanding the body’s response to this novel infectious disease.”Key to their approach was that “rather than focusing solely on drugs that could affect the virus directly, we explored ways to inhibit the cellular processes that the virus uses to infect human cells,” she said. Benevolent’s research findings were published in The Lancet in early February and again twice in the Lancet Infectious Diseases journal.
Post COVID-19: Clinical Trials Will Never Be The Same
For years, technology and software companies, as well as CROs, have been pitching the benefits of patient-centric technologies to sponsor companies. Although a small number of pharma companies have been early adopters of these technologies, others seemed to be taking a wait-and-see approach. COVID-19 has changed that.
We are witnessing a transformation of the clinical trial process occurring at hyper speed. For the last 5 or 10 years, some solutions have been deemed too risky for pharma. Now the risk of not implementing them has become greater than doing so. Right now, it is hard to envision the extent of the changes that are going to occur.