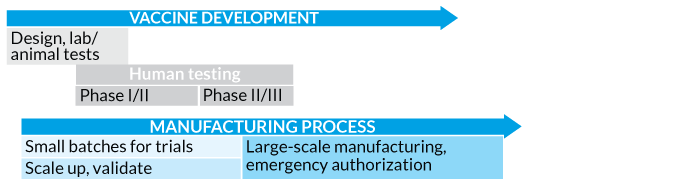
Speed it up The Coalition for Epidemic Preparedness Innovation proposed a truncated process for COVID-19 vaccine development that replaces the lengthy, traditional linear sequence for testing, producing and licensing a vaccine (top). The new approach (bottom) involves running many steps in parallel, including ramping up manufacturing, even before knowing the vaccine will work.
Traditional Vaccine Development
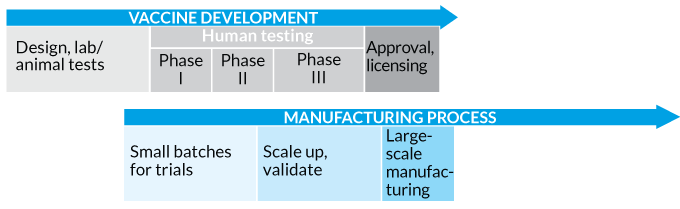
Pandemic Pace
Bicycles are the Latest COVID-Related Shortage
If your bike search has ended in empty store shelves, your best bet right now is visiting an independent local bike shop where, yes, you will pay more, but you will get better quality and much better service than at a big-box store.Jason Reser is owner of Reser Bicycle on Monmouth Street in Newport, Kentucky.He says demand has exploded since mid-March, when people were forced to stay home. Being considered an essential business (transportation), and remaining open didn’t hurt either.
“We’ve been getting phone calls, two or three a day, from California, Texas, the East Coast. Basically, some cities are completely out of bikes, and people are calling us,” he said.The reason, he says: people avoiding mass transit, as well as wanting a family activity during the pandemic.
Gilead ups its Donation of the Covid-19 Drug Remdesivir for U.S. Hospitals
Gilead Sciences, the drug company behind the experimental Covid-19 therapy remdesivir, has upped the number of doses it’s donating to the federal government from 607,000 to around 940,000, STAT has learned.
The new number appeared, with no acknowledgement of the shift, in a letter that a U.S. Department of Health and Human Services official sent to governors on Saturday.
Then, on Monday, in a call with leaders of physician and hospital groups, the HHS assistant secretary for preparedness and response explained that the federal agency would receive additional doses of the drug from Gilead in June, according to two participants on the call. Both asked not to be named to ensure continued access to future HHS calls. One said that at least some of the drug would be in a form useful for treating children.