In the fight against COVID-19, it has become clear that a vaccine is the best long-term solution to quell the pandemic. But what steps need to be taken to develop such a vaccine?
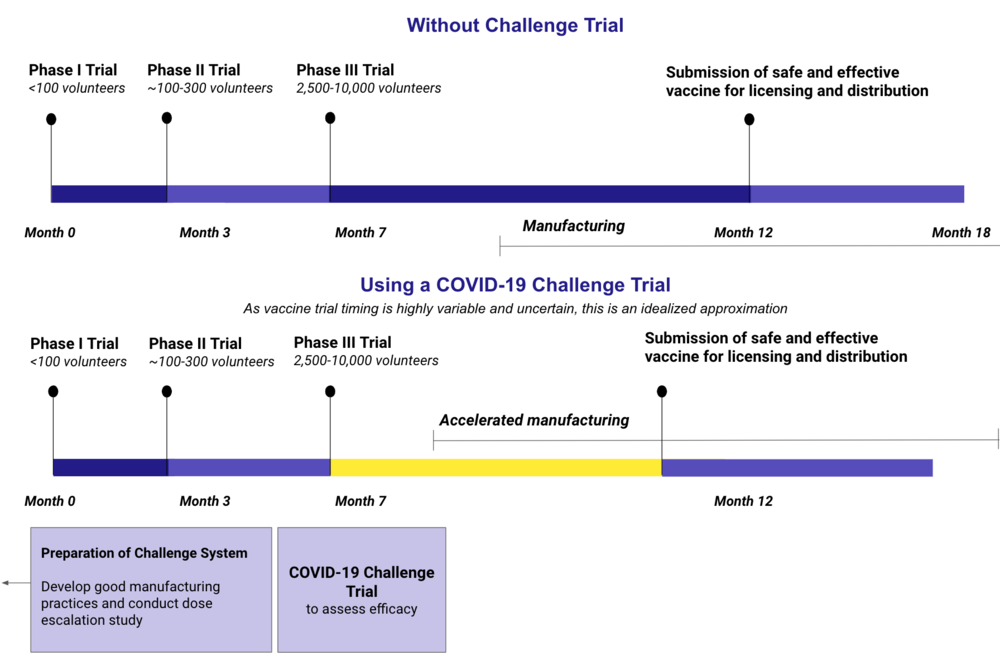
In a typical situation, scientists begin the process of creating a vaccine by conducting extensive research and analysis to develop a prototype. The prototype is then put through four phases of clinical trials, the last of which requires that the test vaccine be administered to thousands of volunteers. During these trials, researchers must wait for volunteers to become infected with the virus in order to evaluate the efficacy of the vaccine. The fourth trial phase alone can take several years to complete. After all trial phases have been completed, the FDA will continue to evaluate different aspects of the vaccine before making it available to the public. For most novel viruses, this process can take between ten and fifteen years.
When Will the Vaccine be Ready?
In the case of COVID-19, researchers all over the globe are working to develop a vaccine. Recent estimates posit that the earliest these could be available to the public would be within a year to a year and a half. While this period is considerably shorter than the wait for many other vaccines, experts are still looking for ways to speed up the testing process. One way that many are now looking to accomplish this is through a human challenge trial. A human challenge trial, or HCT, would involve administering a test vaccine to volunteers and then deliberately infecting them with coronavirus.
Human challenge trials are nothing new, having been conducted in the past to develop vaccines for diseases such as dengue, smallpox, and zika. While there are currently no plans for a human challenge trial in the US, many are advocating for such a study to take place. For instance, 35 members of congress have already signed a document urging regulators to consider approving the use of HCTs, and the World Health Organization has begun to put forward criteria for an ethically acceptable human challenge trial. But the most notable example of support of HCTs is a group called 1 Day Sooner. As of July 2020, the advocacy website has garnered over 30,000 supporters across 140 countries who would be willing to be infected by coronavirus. Because no plans for an HCT have yet been made in the U.S., the sites many volunteers are under no obligations to follow through with their offers.
If proposals for human challenge trials are approved in the future, all possible measures would be taken to ensure the safety of volunteers. The virus being administered would be a specifically developed strain that would not cause severe illness. Volunteers would also be screened to ensure that they were in a low risk age group and had no underlying health conditions. All participants would also be closely monitored after being subjected to the virus and would have access to excellent healthcare.
Sources